The information contained in the site is intended for Swiss healthcare professionals only. By selecting “Okay” below, you verify that you are a licensed Swiss healthcare professional.
By selecting "Okay" below, you verify that you are a licensed CH healthcare professional.


Despite a landscape clouded in complexity, emerging biomarkers are expanding our view of patient populations, and biomarker testing could provide a more comprehensive patient profile.


CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2, isoform IIIb; HER2=human epidermal growth factor receptor 2; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
AN UNMET NEED
In the United States, approximately 6% of patients with metastatic G/GEJ cancer survive 5 years post diagnosis.1,2*†

*US SEER 22 areas (2013-2019), gastric and esophageal cancers, distant stage.1,2
†SEER data do not have a separate classification for GEJ apart from esophageal cancer; therefore, true GEJ projections are unknown.2
IN THE UNITED STATES
In 2023, an estimated 26,500 new cases of gastric cancer (62% advanced‡ stage) and ~21,600 new cases of esophageal† cancer (72% advanced‡ stage) will be diagnosed in the US.1-3
In the US, patients with advanced disease at diagnosis will likely have a poor outcome,4,5 and less than 50% will receive second-line therapy for mG/GEJ cancer.6§
‡Locally advanced (stage II and III) and metastatic (stage IV) gastric/GEJ cancer per tumor node metastases (TNM) staging classification as described in NCCN Guidelines.4,5
§Data from a retrospective analysis of electronic medical records of 3850 eligible G/GEJ/esophageal adenocarcinoma patients that underwent first-line therapy and were alive at 45 days after completion of first-line therapy.6
TNM=tumor node metastases.
AROUND THE WORLD
Over 1.6 million new cases of gastric and esophagealII cancers were diagnosed worldwide in 2020, making them the 5th and 7th most diagnosed cancers, respectively.7
An estimated 1.3 million people died worldwide in 2020 due to gastric and esophagealII cancers, making them the 4th and 6th most deadly
cancers, respectively.7
IIBased on GLOBOCAN 2020 data for gastric and esophageal cancers. GLOBOCAN data do not have a separate classification for GEJ apart from esophageal cancer; therefore, true GEJ projections are unknown.7
EMERGING BIOMARKERS
help identify previously undefined subsets of patients:
ESTABLISHED BIOMARKERS
are used to inform clinical decisions:
CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2, isoform IIIb; HER2=human epidermal growth factor receptor 2; MMR=mismatch repair; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.

CLDN18.2
Claudins are a family of transmembrane proteins8:
Claudins are a major component of tight junctions, which are involved in controlling the flow of molecules between cells.8,9
Claudins are present throughout the body, but two specific isoforms of CLDN18 are localized to certain tissue types8,17:
Preclinical data have shown that CLDN18.2 may become more exposed
as gastric tumors develop.8,16
CONFINED IN HEALTHY TISSUE
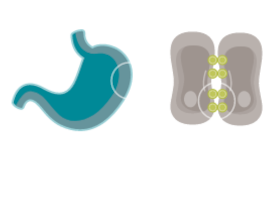

In normal gastric mucosa, CLDN18.2 is typically buried within tight junctions8,16
RETAINED AND EXPOSED IN MALIGNANT TRANSFORMATION
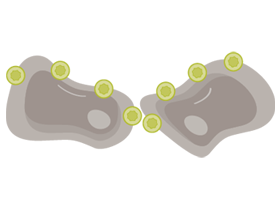

CLDN18.2 is often retained during malignant transformation.
CLDN18.2 may be more exposed when cell polarity disruptions and structure loss occur.8,16,18
MAINTAINED IN METASTATIC PROGRESSION
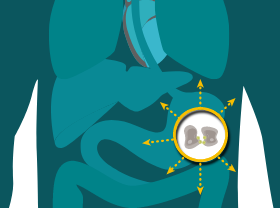

CLDN18.2 may also be localized in lymph node metastases of gastric adenocarcinoma as well as other distant metastatic sites.8,19-21
The information provided above is based on the current understanding of data.
CLDN18.2 expression may also be observed in esophageal adenocarcinoma, pancreatic adenocarcinoma, non-small cell lung cancer, and ovarian mucinous adenocarcinoma.8
While approximately 70% of advanced G/GEJ cancers express CLDN18.2 (at any detectable amount),20* two recent studies have shown that approximately 38% of patients with locally advanced unresectable or mG/GEJ cancer are Claudin18.2 positive (≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC).22,23†
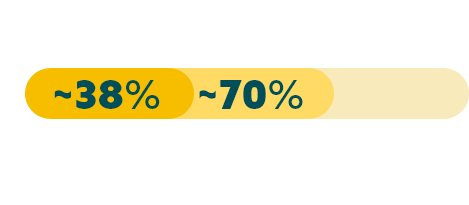

*Data from a retrospective analysis of 350 Caucasian patients with advanced G/GEJ cancer.20
†Data from 2 global randomized Phase 3 studies: the first study included 2,403 assessable patients, of which 922 were CLDN18.2 positive; and a second study which included 2,104 assessable patients, of which 808 were CLDN18.2 positive.22,23
‡Any detectable amount: moderate to strong membranous CLDN18 staining by immunohistochemistry (IHC) in any percentage of tumor cells.20
Biomarker prevalence estimates from select studies are reported below. Prevalence data can vary among studies due to tumor heterogeneity, differences in patient population, clinical trial methodology, and diagnostic assays used. Positivity thresholds also vary by study.13,22-24,26-28
EMERGING BIOMARKERS
CLDN18.222,23
(positive)§
38%
FGFR2b26||
(positive)
30%
§≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC.22,23
||FGFR2b positivity: FGFR2b overexpression (IHC 2+/3+ any amount of tumor cells) and/or FGFR2 gene amplification by ctDNA (NGS 1.5x increase in FGFR2).26
ESTABLISHED BIOMARKERS
PD-L124,27
(variable due to multiple factors)¶
CPS ≥1: 67-73%
CPS ≥5: 29-31%
CPS ≥10: 16-18%
HER213
(positive)
22%
MSI28
(MSI-high)
4%
CPS=combined positive score.
¶ PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomized controlled trial and a real-world retrospective medical records study.24,27
EMERGING BIOMARKERS
CLDN18.2:
IHC22,23
FGFR2b:
IHC, NGS (ctDNA)26#
#FGFR2b protein overexpression assessed by IHC; FGFR2 gene amplification assessed using ctDNA by NGS.26
ESTABLISHED BIOMARKERS
PD-L1:
IHC4,5**
HER2:
IHC, ISH, NGS4,5,25††
MMR/MSI:
IHC, PCR/NGS4,5‡‡
ctDNA=circulating tumor DNA; IHC=immunohistochemistry; ISH=in situ hybridization; NGS=next generation sequencing; PCR=polymerase chain reaction.
** Varying diagnostic assays.29
†† Other ISH methods (FISH=fluorescent ISH; SISH=silver ISH; CISH=chromogenic ISH; DDISH=dual-color dual-hapten ISH).25
‡‡ MMR assessed by IHC, MSI by PCR/NGS.4,5
IHC scoring for CLDN18 in G/GEJ cancer is performed based on membrane staining intensity and percent of positive tumor cells22,23,30
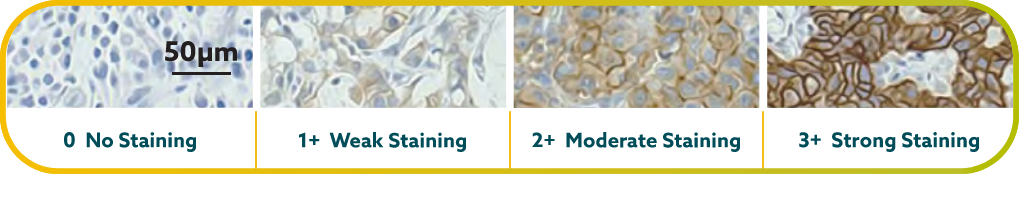
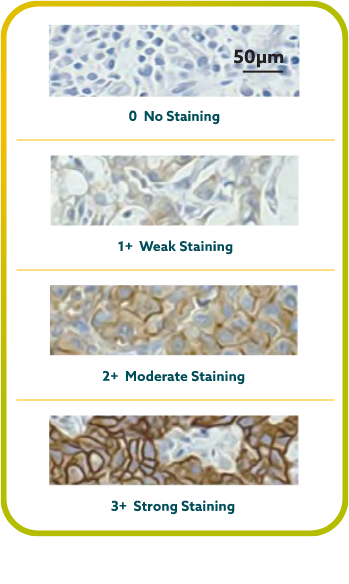
CLDN18.2 positivity is defined as ≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC.22,23
CLDN18.2 expression has been seen in both diffuse-type gastric tumors and intestinal-type gastric tumors.20

FGFR2b
FGFR2b participates in angiogenesis and cell proliferation through
FGFR signaling pathways.11,12
FGFR2b positivity has been observed in 30% of advanced G/GEJ cancers.26*


FGFR2b positivity: FGFR2b overexpression (IHC 2+/3+ any amount of tumor cells) and/or FGFR2 gene amplification by ctDNA (NGS 1.5x increase in FGFR2)
*Data from select studies.26
Detecting FGFR2b can be done with the following tests26:

HER2
HER2 (human epidermal growth factor receptor 2) is a receptor-tyrosine kinase that is overexpressed and/or amplified in advanced G/GEJ cancer.11
HER2 is a proto-oncogene that is involved in signaling pathways, which leads to cell growth and differentiation.25
HER2 positivity has been reported in 22% of advanced G/GEJ cancers.13*


HER2 positivity: overexpression (IHC3+) and/or gene amplification (FISH-positive)
*Data from select studies.13
Detection of HER2 may be done with IHC, NGS, and ISH methods.4,5
MSI
MSI is associated with genomic instability and increased susceptibility to tumor development.11
Microsatellites are repeated sequences of nucleotides in DNA.14
MSI-H has been reported in 4% of advanced G/GEJ cancers.28*


*Data from select studies.28
Detection of MSI and MMR is typically assessed with various methods.4,5

PD-L1
PD-L1 (programmed death-ligand 1) is a transmembrane protein that may be expressed on various tumor cells and/or immune cells.37
Prevalence of PD-L1 has been reported for several positivity thresholds throughout various studies24,27*:






PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomized controlled trial and a real-world retrospective medical records study.24,27
*Data from select studies.24,27
PD-L1 expression is detected using IHC.4,5
CPS=combined positive score.
SUMMARY
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer and Esophageal and Esophagogastric Junction Cancers* support using biomarkers to help map the path forward for patients.4,5†
The NCCN Guidelines® recommend4,5:
*In adenocarcinomas of unresectable locally advanced, locally recurrent or metastatic disease of esophageal and esophagogastric junction cancers.5
†This is a summary of relevant portions of the NCCN Guidelines. Please see the full NCCN Guidelines for Gastric Cancer and Esophageal and Esophagogastric Junction Cancers at NCCN.org.4,5
Biomarker testing provides more insight into advanced G/GEJ cancer as more biomarkers are discovered.
‡The use of IHC/ISH/targeted PCR should be considered first, followed by additional NGS testing as appropriate.4,5
§Data from select studies. Prevalence data can vary among studies due to tumor heterogeneity, differences in patient populations, clinical trial methodology, and diagnostic assays used. Positivity thresholds may vary by study.13,24,26-28
As biomarker research continues, it expands our view of the patient population, reveals more information about the advanced G/GEJ cancer landscape, and helps inform clinical decisions.
Stay Up To Date
REFERENCES